Biodefense
Treatment of infections caused by the deliberate release of pathogens
Finafloxacin shows remarkable activity against a number of the highly dangerous category A and B biological agents, as classified by the Centers for Disease Control and Prevention (CDC), especially Burkholderia pseudomallei, Burkholderia mallei, Francisella tularensis, Coxiella burnetii and Yersinia pestis. MerLion is collaborating with the UK Defence Science and Technology Laboratory (Dstl) - in a program funded by US Defense Threat Reduction Agency (DTRA) - to determine the in vitro and in vivo activity of finafloxacin against different pathogens that are considered to be potential bioweapons.

Finafloxacin has proven activity against a broad range of biothreat pathogens
Finafloxacin has demonstrated efficacy against infections caused by a range of biodefense agents including B. pseudomallei and F. tularensis in murine models of disease. Such pathogens are highly infectious and can cause severe illness in exposed individuals. They are especially dangerous as they can be easily cultivated, stored, or transported under standard conditions. They could therefore be deliberately released by spreading them among the population.
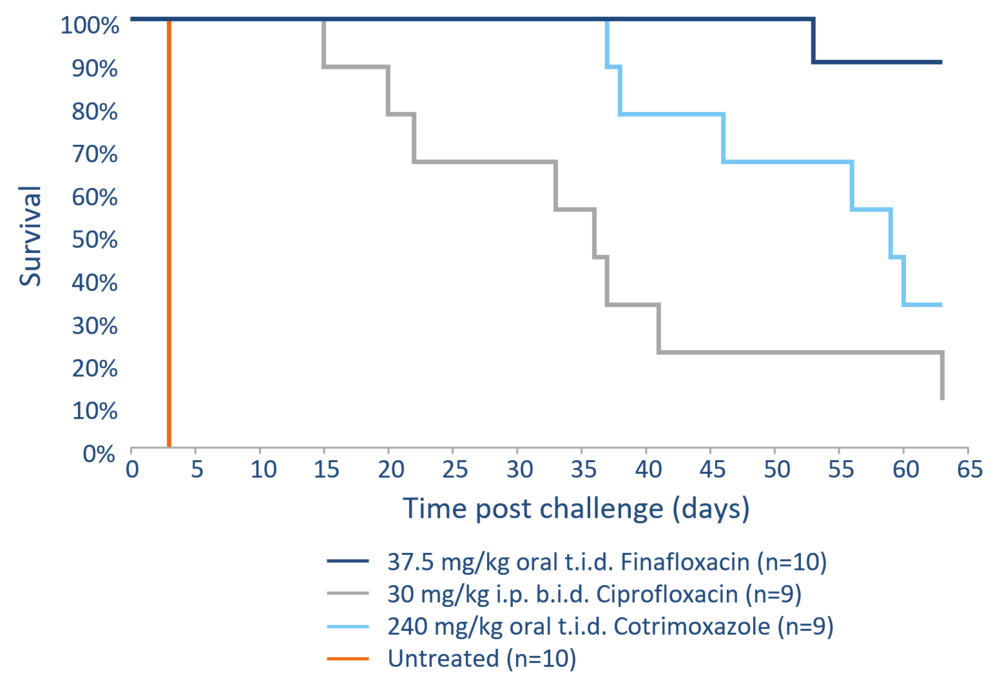
The development of efficient drugs against these pathogens is therefore of particular importance. The broad efficacy against such pathogens makes finafloxacin an outstanding option for prophylaxis as well as treatment following exposure to such biothreat pathogens, in particular as systemic treatment options (oral, iv) for finafloxacin are readily available. The efficacy of finafloxacin against B. pseudomallei was evaluated in an inhalational murine model of melioidosis with ciprofloxacin and cotrimoxazole as comparators.
B. pseudomallei is a soil-dwelling gram-negative bacterium and the causative agent of melioidosis, a serious disease endemic in Southeast Asia and Northern Australia. Antibiotic treatment is lengthy and relapse often occurs. The disease is a growing problem in the affected countries and resistance against treatment options like cotrimoxazole have been reported.
Finafloxacin efficacy against B. pseudomallei was evaluated in an inhalational murine model of melioidosis with the comparators ciprofloxacin and cotrimoxazole. Following the determination of the pharmacokinetic profile in mice and matching of the AUC/MIC with the human pharmacokinetic profile, an equivalent dose of finafloxacin was administered to the mice. After an initial phase of protection, the majority of the mice treated with ciprofloxacin and cotrimoxazole succumbed to the infection whereas 90% of the mice treated with finafloxacin survived even 63 days after the start of the study. Finafloxacin provided high protection against an inhalational challenge with B. pseudomallei suggesting that it could provide a benefit when used as a treatment or prophylaxis against inhalational melioidosis, especially in the event of a deliberate release or accidental exposure.