Overview
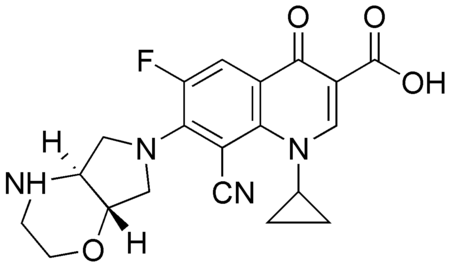
MerLion is developing finafloxacin, a novel C-8-cyano 5th generation fluoroquinolone, for human use. Systemic and topical formulations of finafloxacin have passed the different development stages, including the completion of clinical phase II. In preclinical and clinical studies, involving more than 1,000 subjects overall, finafloxacin has shown none of the toxicity aspects of other, earlier-generation quinolones. Finafloxacin displays an outstanding safety and tolerability profile even with dose levels of up to 1 g/day iv administered for 7 days.
The US Food and Drug Administration (FDA) designated the oral and iv formulations of finafloxacin as a Qualified Infectious Disease Product (QIDP). This includes fast track development status for complicated urinary tract infections (cUTIs) including pyelonephritis, complicated intraabdominal infections (cIAIs) and acute bacterial skin and skin structure infections (ABSSSIs). MerLion has successfully completed a phase II clinical study in cUTI patients with finafloxacin and is currently preparing cUTI phase III clinical studies. Furthermore, the US FDA and Health Canada already approved the use of finafloxacin as a topical medication (XtoroTM) for the treatment of acute otitis externa (ear infections, "swimmer's ear").
Mode of action
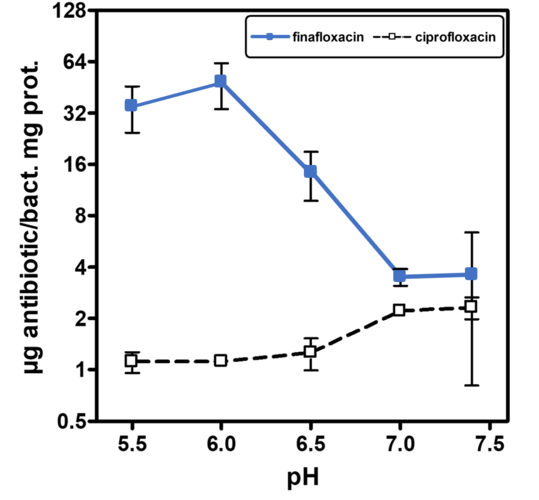
Finafloxacin inhibits DNA synthesis by promoting cleavage of bacterial DNA in the DNA-enzyme complexes of DNA gyrase and topoisomerase IV, resulting in bacterial death. Its high dual target activity contributes to its very rapid bactericidal activity, even in stationary phase or in growth arrested cultures. The concentration of finafloxacin required to achieve 50% DNA cleavage was 12 or 25-fold lower, respectively, than that of other FQs, resulting in substantially increased potency. Finafloxacin has also displayed vastly superior activity in numerous in vitro and in vivo studies with biofilms, slowly growing bacteria, small colony variants and intracellular pathogens. Recent studies have demonstrated that finafloxacin rapidly enters and accumulates in cells, while only effluxing slowly out of the cells again at low pH.
Enhanced activity in an acidic environment
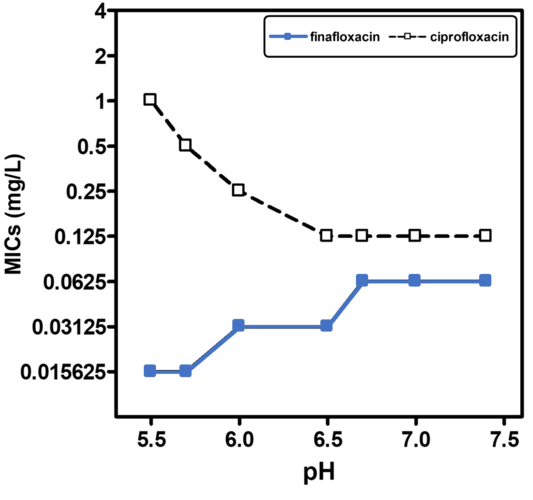
A low pH is often found in infection sites and is also relevant for intracellular pathogens, which can reside in the acidic organelles or subcellular compartments of host cells. It is therefore physiologically relevant and clinically important to study the activity of antibiotics at low pH to mimic relevant conditions more closely, particularly as fluoroquinolones are amphoteric molecules whose antibacterial activity is influenced by pH. All currently marketed quinolones (as well as other antibiotic classes) are significantly less active at acidic pH (5.0 - 6.0) than at neutral pH. Finafloxacin differs from these in that its activity is substantially increased under acidic conditions, due to its higher accumulation within the cell at low pH while also being a poor substrate for bacterial multidrug efflux transporters.
In vitro activity
Finafloxacin has demonstrated broad spectrum activity against a wide range of organisms, including Gram-positives, Gram-negatives and anaerobes. A recent study including 18 bacterial species, confirmed an increase in the activity of finafloxacin at acidic pH (5.8 to 6.2), compared to neutral pH whereas other fluoroquinolones lost activity. Compared to other fluoroquinolones finafloxacin also showed excellent activity against several anaerobes. Finafloxacin also demonstrated bactericidal activity against adherent and planktonic Escherichia coli, when compared to ciprofloxacin and levofloxacin. Similar results have been obtained in biofilm models with other pathogens.
Preclinical and clinical safety
Finafloxacin has shown an outstanding safety profile in preclinical safety studies in animals (e.g. rats and dogs). In addition, the safety and tolerability of oral and/or iv formulations of finafloxacin has been investigated in five phase I and three phase II clinical trials, with a total of 414 study participants. In these studies, single daily oral or iv doses of up to 1,000 mg were successfully evaluated. Reported adverse events, mostly headache and diarrhea, were generally mild. There were no clinically relevant changes in vital signs, routine laboratory tests or ECG associated with the administration of finafloxacin. The pharmacokinetic evaluation showed a linear increase of terminal half-life, maximum concentration and AUC for both iv and orally administered finafloxacin. Finafloxacin is well absorbed, with a 75% bioavailability when administered orally (800 mg, once daily).
High pharmaco-economic benefit
Due to its superior, fast-acting efficacy finafloxacin leads to quicker relief from pain, earlier discharge and fewer treatment failures. Moreover, the drug offers the option to easily switch from iv to oral administration. Finafloxacin was granted QIDP and fast track status by the US FDA until July 2013 for cUTI including pyelonephritis, cIAI and ABSSSI, which provides excellent economic opportunities.
Overcoming resistance
Finafloxacin is active against multi-drug resistant (MDR) bacterial strains, where other fluoroquinolones displayed only limited activity. Evaluated against a panel of ciprofloxacin-resistant Acinetobacter baumannii strains, finafloxacin showed comparable activity at neutral pH and superior activity under acidic conditions. Finafloxacin was also more active than other fluoroquinolones against methicillin-resistant, vancomycin-resistant, linezolid-resistant and daptomycin-non-susceptible Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis, at both neutral and acidic pH. Superior susceptibility in comparison to that of other fluoroquinolones is furthermore a result of finafloxacin being a poor substrate of the major facilitator superfamily multidrug efflux transporters like NorA in S. aureus and or Lde in Listeria monocytogenes, the MDR efflux pump QepA1 in E. coli, and the BpeEF-OprC efflux pump in Burkholderia pseudomallei.
Activity against resistant S. aureus (MRSA)
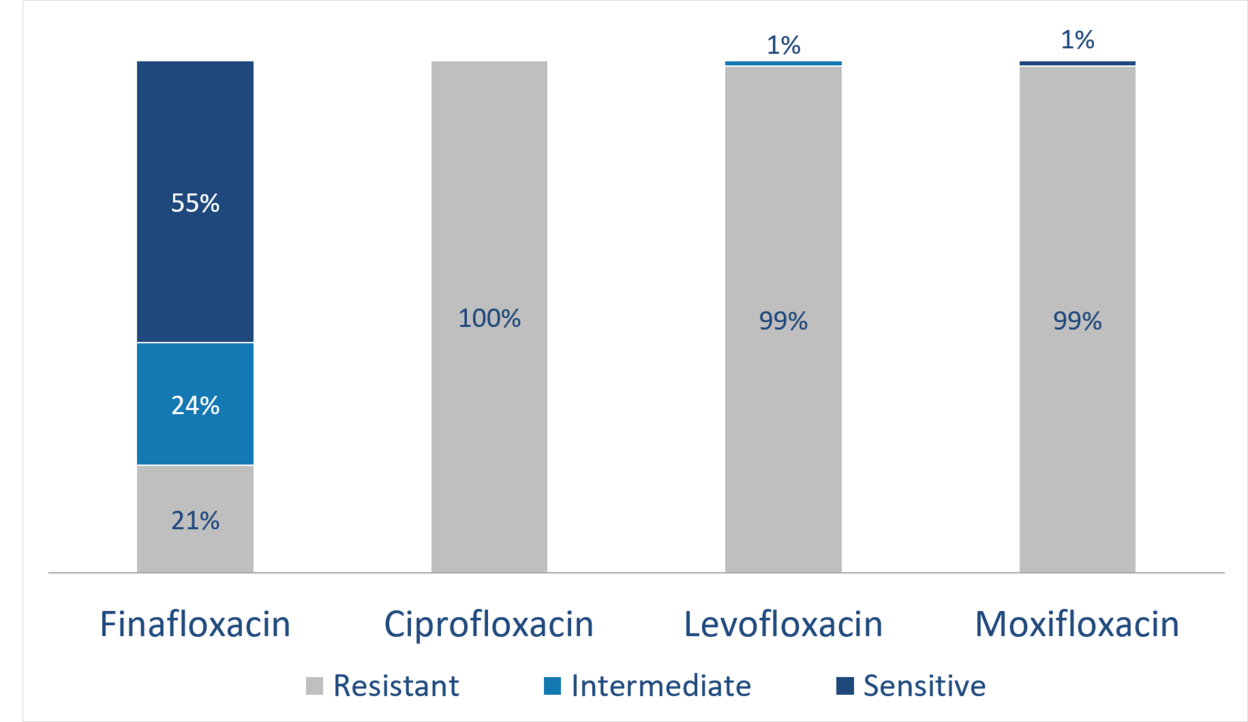
Unmatched efficacy
In phase I, II and III trials, finafloxacin has shown activity as a fast-acting antibiotic directed against a broad spectrum of bacteria, even multi-resistant bacteria, such as MRSA and ciprofloxacin-resistant staphylococcus species. Of note, finafloxacin is also active against slow-growing, persistent bacteria, including biofilms. Moreover, finafloxacin shows increased activity in an acidic environment, a condition relevant in various important indications.
Xtoro™
Finafloxacin for the treatment of acute otitis externa
FDA approved product - ready for commercialization
- Xtoro™ is MerLion’s US FDA and Health Canada approved drug for the treatment of acute otitis externa (AOE) with finafloxacin
- Xtoro™ is a topical liquid suspension formulation of finafloxacin
Biodefense
Finafloxacin for the use against biothreats
Promising candidate as countermeasure for biothreat pathogens
- MerLion's activities with finafloxacin as drug for biodefense purposes are supported by the UK Ministry of Defence and funded by the US Defense Threat Reduction Agency
- Finafloxacin has shown strong activity against nearly all bacteria classified as category A or B biothreads by the Centers of Disease Control and Prevention
- Type C meeting with the US FDA in October 2018 to evaluate the option to develop finafloxacin for defense against biowarfare pathogens
cUTI
Finafloxacin for the treatment of cUTI
Developing finafloxacin for hospital indications
- Intravenous and oral formulations provide a unique opportunity for a short course (5 days) with once daily systemic dosing
- Phase III-ready: After successful completion of phase II, finafloxacin has the potential to be developed in phase III for the use in cUTI and other hospital indications