Complicated urinary tract infections
Finafloxacin is a novel candidate for the treatment of multiple, life-threatening infection indications, like complicated urinary tract infections (cUTIs). Coming from a well-known and well understood “workhorse” class of antibiotic, its unique properties in activity, safety and flexible dosing regimen options, clearly distinguish finafloxacin from other, existing therapies, offering new and outstanding potential treatment opportunities.
cUTIs account for a large part of antibiotic administrations and also belong to the most common hospital acquired infections. A low pH, acidic environment is often the physiological consequence of a bacterial infection and also very frequent in the urine of patients suffering from cUTI. Under these conditions, a number of approved antibiotics including other marketed fluoroquinolones significantly lose activity. Finafloxacin, however, comes with the unique property of having its already high activity significantly further enhanced under acidic conditions, making it the ideal choice to treat this kind of infection.
Key advantages of finafloxacin for a use in cUTI
High antibacterial activity
Broad spectrum of activity, also against uropathogens, including fluoroquinolone-resistant, multiresistant or extended-spectrum beta-lactamase (ESBL) producing strains.
Daily single dose
The safety and pharmacokinetic profile of finafloxacin supports a high once daily single dose / short-course treatment regimen.
High urinary titers
Large amounts of finafloxacin are excreted via the urine, leading to high urinary bactericidal titers in the genitourinary system, the site of the actual infection.
Faster pathogen eradication
Various studies show a quicker and more efficient killing of key urinary pathogens by finafloxacin compared to other currently marketed fluoroquinolones and other antibiotics, especially in the acidic environment found in the urine.
Activity against slowly growing pathogens
Finafloxacin has proven activity against slowly growing bacteria and biofilms often found in catherterized cUTI patients.
Carbapenem sparing
Finafloxacin has the potential to be ‘carbapenem sparing’ and saving other novel antibiotics targeting multidrug resistant pathogens.
Formulations for oral and iv administration
The available finafloxacin ready-to-use iv solution and tablets enable initial iv treatment of patients in the hospital and, after release from immediate care and hospital, subsequent oral treatment at home.
Clinical data for cUTI
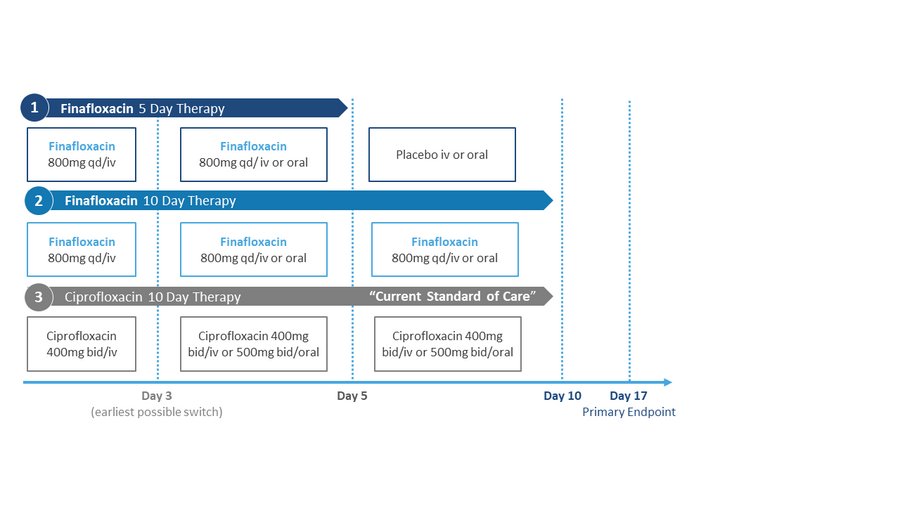
MerLion has conducted a blinded, active-controlled and randomized phase II with 225 patients (intention-to-treat population, ITT) suffering from cUTI or acute pyelonephritis in Germany and Poland with a total of 193 evaluable patients in the microbiological-intention-to-treat population (m-ITT). The primary objective of the study was evaluate the microbiological and clinical outcome of treatment with finafloxacin for 5 days versus finafloxacin for 10 days versus ciprofloxacin for 10
days as a reference comparator. Male and female patients who had cUTI or pyelonephritis requiring a hospitalization were eligible for the study. Only patients with confirmed uropathogens were included in the m-ITT population.
The patients were randomized to one of three groups receiving either finafloxacin iv followed by finafloxacin oral for 5 days, finafloxacin iv followed by finafloxacin oral for 10 days or ciprfloxacin iv followed by ciprofloxacin oral for 10 days. The earliest day for switching from iv to oral was on day 3.
Phase II clinical study design
The urine analysis of each patient after three days of treatment with finafloxacin showed already high levels of bacterial eradication in general and a higher eradication rate than with ciprofloxacin. The higher efficacy of finafloxacin compared to ciprofloxacin at this early point in time can thereby be attributed to the higher antibiotic activity in the usually acidic urine and the more efficient killing of fluoroquinolone resistant pathogens by finafloxacin found in these patients.
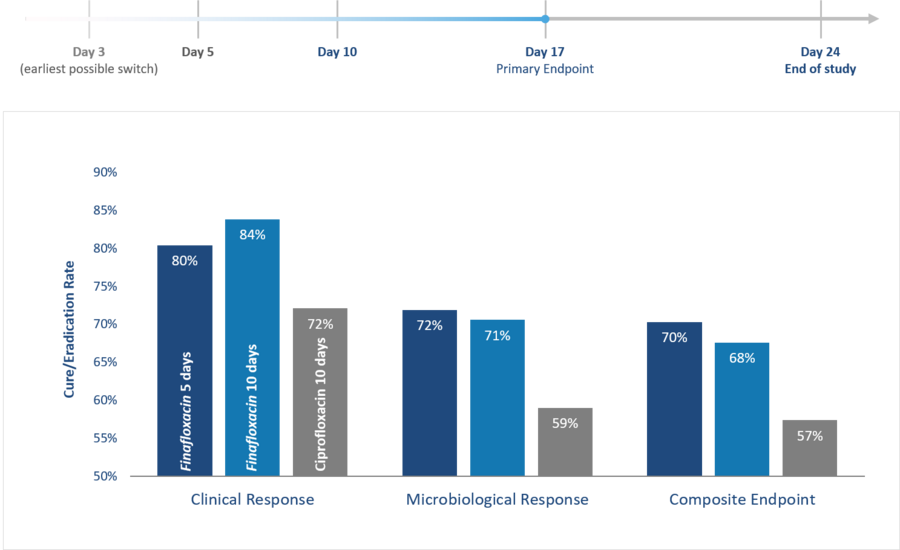
These results were confirmed at the primary endpoint of the study (day 17 after treatment initiation); fewer patients treated with finafloxacin showed signs and symptoms of the disease (clinical response) and the eradication of pathogens from their urine was more efficient (microbiological response). Accordingly, the patients treated with finafloxacin had a superior outcome in the composite endpoint (combined clinical and microbiological response).
Furthermore, he results at the primary endpoint of the study at day 17 indicated that the shorter, 5-day, once daily, single dose treatment with finafloxacin was already sufficient for a successful cure from the infection. Requiring patients to be treated for 10 days, often today's standard clinical practice with the currently available medication, appears not to be needed with finafloxacin. An additional benefit of such a potentially short, 5-day regimen constitutes an additional benefit of finafloxacin against its comparators in that it minimizes the chances for the emergence of drug resistances to manifest.
Phase II study results in cUTI
Primary endpoint at day 17